New Publication: Improving cell-based therapies for intervertebral disc degeneration: Enhancing the culture conditions of spine cells improves their regenerative capacity
Chronic Lower back pain (LBP) is the leading cause of disability and morbidity worldwide. Degeneration of the spine’s intervertebral discs (IVDs) is a common cause of chronic LBP and accounts for about 40% of all cases. IVDs are structures that are embedded in the spine, and when healthy they provide cushioning during movement. They are made of special tissue-specific cells including nucleus pulposus cells. Previous research shows that adding healthy nucleus pulposus cells to the degenerated IVDs could promote regeneration.
However, two problems exist when it comes to using them. Firstly, nucleus pulposus cells are scarce. This means that extensive expansion of these cells outside of the body is required to obtain enough cells for insertion into the degenerated IVDs. Secondly, once obtained, cells adapt to the culture conditions outside the body and can lose their capacity for IVD regeneration. Researchers can determine this ‘loss of fitness’ by characterizing several known markers which are expressed on the outside and inside of these cells (this characterizes their phenotype).
A promising strategy to optimize the expansion of these cells outside of the body is to focus on factors that influence the nucleus pulposus cell’s phenotype. Studies in humans show that increasing the concentration of particles dissolved in the lab’s culture medium – in other words increasing osmolarity – enhances the phenotype and regenerative capacity of nucleus pulposus cells. However, this has not been tested on dog NPCs even though dogs also suffer from LBD due to IVD degeneration and they represent a clinically relevant model for IVD regeneration in humans.
In this study, Lisanne Laagland and colleagues collected nucleus pulposus cells from dog spines showing mild degeneration of IVDs. These cells don’t have the optimal phenotype for IVD regeneration and can therefore be seen as unhealthy. By expanding these cells in culture media with varying osmolarities they aimed to
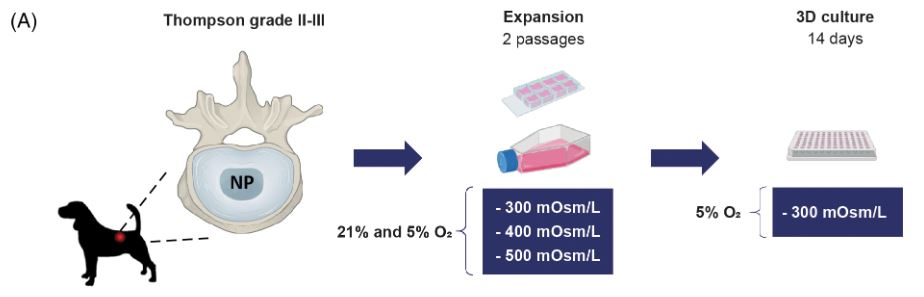
Study design
improve their phenotype and with that their regenerative capacity.
They found that these optimized expansion conditions indeed improved the phenotype of the dog nucleus pulposus cells, indicating that cells grown this way could be more suitable to regenerate the spine’s IVDs.
Taken together these results are an important piece of the puzzle to find an optimal treatment for chronic LBP in the future. By determining the optimal culture conditions of IVD cells, researchers can expand these precious cells more efficiently, leaving them more capable of regenerating IVD tissue.
However, the road to an efficient cell-based treatment is still long and several obstacles need to be overcome before both human and dog patients in the clinic can actually benefit from this treatment.
Read about other cell based therapies here!
Hyperosmolar expansion medium improves nucleus pulposus cell phenotype
Lisanne T. Laagland, Frances C. Bach, Laura B. Creemers, Christine L. Le Maitre, Deepani W. Poramba-Liyanage, Marianna A. Tryfonidou
First published : 18 August 2022
Abstract:
Background: Repopulating the degenerated intervertebral disc (IVD) with tissue-specific nucleus pulposus cells (NPCs) has already been shown to promote regeneration in various species. Yet the applicability of NPCs as cell-based therapy has been hampered by the low cell numbers that can be extracted from donor IVDs and their potentially limited regenerative capacity due to their degenerated phenotype. To optimize the expansion conditions, we investigated the effects of increasing culture medium osmolarity during expansion on the phenotype of dog NPCs and their ability to produce a healthy extracellular matrix (ECM) in a 3D culture model.
Methods: Dog NPCs were expanded in expansion medium with a standard osmolarity of 300 mOsm/L or adjusted to 400 or 500 mOsm/L in both normoxic and hypoxicconditions. Following expansion, NPCs were cultured in a 3D culture model in chon-drogenic culture medium with a standard osmolarity. Read-out parameters included cell proliferaton rate, morphology, phenotype and healthy ECM production.
Results: Increasing the expansion medium osmolarity from 300 to 500 mOsm/L resulted in NPCs with a more rounded morphology and a lower cell proliferation rate accompanied by the expression of several healthy NPC and progenitor markers at gene (KRT18, ACAN, COL2, CD73, CD90) and protein (ACAN, PAX1, CD24, TEK,CD73) level. The NPCs expanded at 500 mOsm/L were able to retain most of their phenotypic markers and produce healthy ECM during 3D culture independent of the oxygen level used during expansion.
Conclusions: Altogether, our findings show that increasing medium osmolarity during expansion results in an NPC population with improved phenotype, which could enhance the potential of cell-based therapies for IVD regeneration.
Funding information: Dutch Arthritis Society, Grant/Award Numbers: LLP12, LLP22; Horizon 2020 Framework Programme, Grant/Award Number: 825925
Read the full paper here!

