The project will begin in the lab, with the scientific development of viable stem cell and smart biomaterial. These will be combined and tested in a battery of testing platforms that mimic the diseased environment. Finally, cellular and clinically relevant animal models will be used to test for translation and safety, culminating in a proof-of-concept study in canines.
Spanning the entire project, a team of ethicists and regulatory advisors will provide guidance on the project to ensure all ethical and legal considerations and regulatory guidelines are met. In addition, we will be actively communicating and disseminating project information, updates, and results to the public. Finally, the consortium efforts will be facilitated by a team of project managers working to bridge communication across work packages and bring the project to successful project completion.
“The work packages led by experts in the field perfectly complement the challenging iPSpine project. This approach covers all aspects involved in succesful translation of iPS-based therapies from bench to bedside.”
– Marianna Tryfonidou, Project Coordinator, Universiteit Utrecht.
The development of the iPSpine advanced therapy will be carried out through nine different work packages (WPs) as described below.
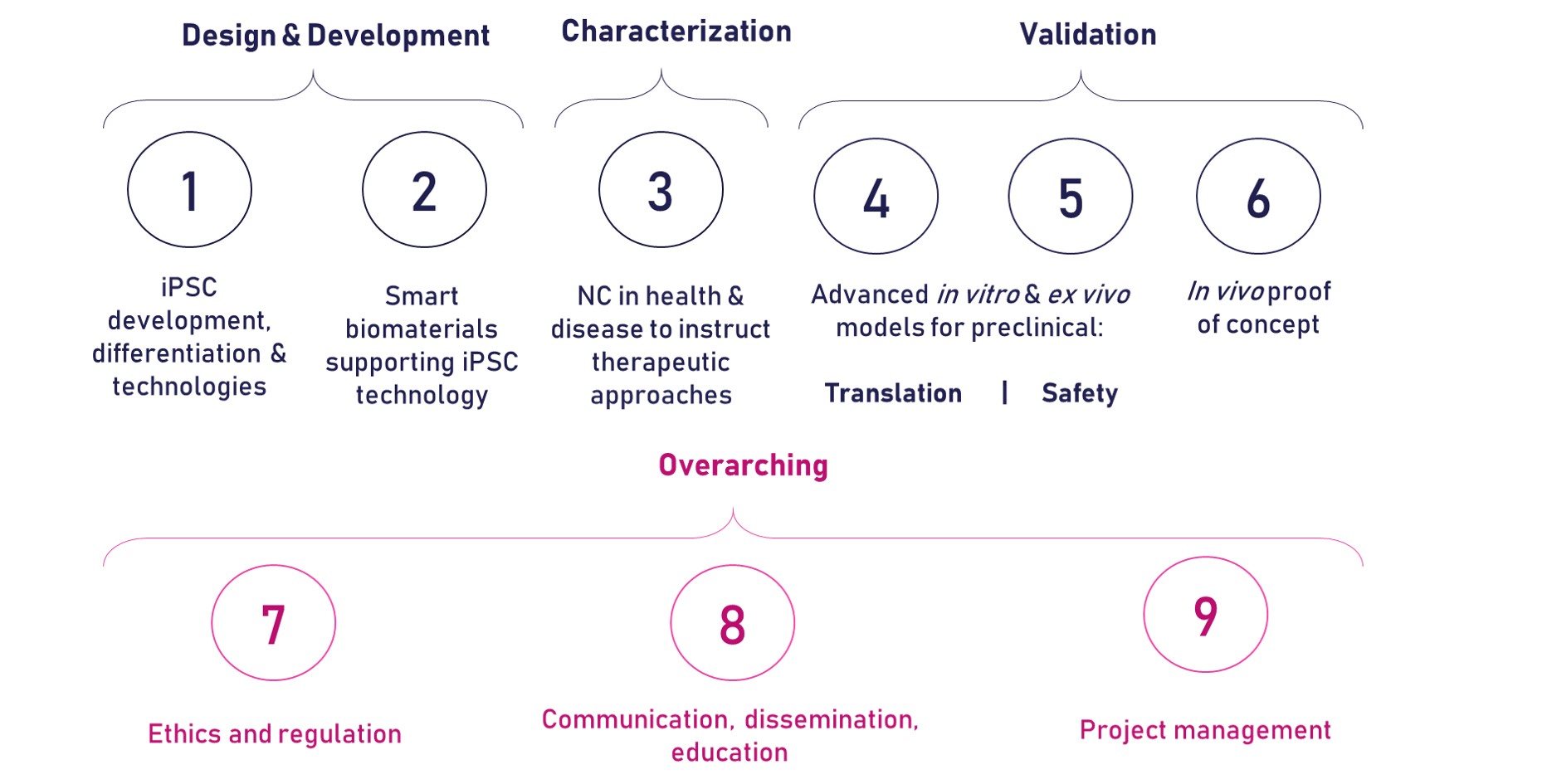
WP1 – iPSC development, differentiation and technologies
We develop and optimize methods to differentiate human induced pluripotent stem cells (iPSCs) into fully mature notochordal-like cells (NLCs) in the laboratory. These NLCs, upon injection, will re-populate and rejuvenate the degenerate and painful disc. To ensure that the developed iPSC line can optimally achieve the aforementioned, different tissue sources are explored:
1. Mononuclear cells which can be obtained from blood;
2. Progenitor cells from the disc. It has been shown that iPSCs maintain a memory of their parental cells which is expected to allow them to better differentiate to NLCs. This technology is being brought to the next level by innovative technological tools that instruct further the iPSCs to become NLCs, including smart biomaterials and intra-cellular delivery of the necessary signals.
WP2 – Smart Biomaterials supporting iPSC technology
The engineered cells need an environment that mimics the native healthy tissue in order to thrive. For this purpose, biomaterials that contain tissue-specific components, and as such can provide such an environment, are further developed in this WP. We use natural, semi-synthetic, and fully synthetic biomaterials that mimic the healthy disc environment. These biomaterials are being developed in such a way that they can provide an instructive micro-environment that stimulate iPSCs to differentiate into NLCs. Additionally, in the biomaterials, the cells can thrive while eliciting beneficial effects on the resident disc cells. Furthermore, these biomaterials act as a vehicle to deliver cells to the disc and simultaneously provide direct mechanical support immediately after application.
WP3 – NC in Health & Disease to instruct therapeutic approaches
Extensive characterisation of the iPS-NLCs from WP1 are performed by the Partners. To facilitate this, an open-access knowledge-sharing platform is being developed to collect, share, and perform integrative bioinformatic analysis. Within the context of the envisioned therapy, the crosstalk between iPS-NLCs and the resident disc cells is studied. For the purpose of translating from bench to bedside, the consortium focuses on the influence that iPS-NLCs have on human disc cells, but also explore the effects on canine disc cells, as dogs are employed for efficacy studies within WP6. With the developed knowledge-sharing platform, the data and knowledge generated in this WP and throughout the iPSpine project can be accessed by the iPSpine partners and other stakeholders across the globe.
WP4 – Advanced in vitro and ex vivo models for preclinical development
To understand and evaluate how a degenerating disc can be regenerated, in vitro models with increasing complexity, and ultimately employing tissue explants, are being developed in order to mimic the pathophysiological and physico-chemical environment of the degenerating disc. These models are used to investigate the regenerative capacity and anti-inflammatory effects of the biomaterials (WP2), combined with the iPS-NLCs (WP1 and WP3). In addition, a miniaturised portable spine tester is being developed to evaluate the biomechanical performance of the regenerated disc tissues. Within WP4, using iPSpine as a model, a high-level management platform is developed that blends the complex processes of pre-clinical development and the respective regulatory aspects. Hereby iPSpine envisions a tool to facilitate informed decisions during preclinical development of therapies of the future (advanced therapy medicinal products: ATMPs).
WP5 – Advanced in vitro and in vivo models for preclinical safety
Short and long-term safety studies are being conducted to assess the purity of the isolated human iPS-NLCs after differentiation and to establish the safety of the proposed cell-based therapeutic strategy (with focus on clinically relevant small and large animal models). In vitro assays determining safety focus on demonstrating purity, genetic stability, and lack of an immune reaction of the iPSC line to be used for in vivo application. Biodistribution and toxicology studies on iPS-NLCs with and without the selected biomaterial are conducted to comply with regulatory aspects related to the iPSpine therapy. Sex and gender-related aspects of safety are addressed within this WP in the in vitro and in vivo setting.
WP6 – In vivo Proof-of-concept
A platform is being developed detailing guidelines to conduct in vivo studies to deliver Proof of concept for the proposed approach of the advanced therapy. Efficacy studies are conducted to demonstrate regenerative and anti-inflammatory properties of iPS-NLC together with the selected biomaterials in an experimental canine model. This is followed by a two-centre clinical trial with canine patients suffering from disc-related low back pain.
WP7 – Ethics & Regulation
WP7 consists of two integrated and complementary streams: 1) an Ethics Stream which centres on ethics and policy aspects, and 2) a Regulatory Stream which focuses on regulatory challenges related to advanced therapies of the future that is applicable to large patient populations. Both streams aim to contribute to ethically sound and responsible stem cell research.
WP8 – Dissemination, Communication, Education
The most important part of the dissemination activity is the timely and appropriate dissemination of the key messages to defined target groups (policy makers and general public; patients and patient advocacy groups; scientific and medical community; investor and pharmaceutical industry). This dissemination will allow 2-way communication facilitating engagement and input of external stakeholders.
WP9 – Project Management
This WP includes overall scientific, legal, financial, and administrative management of the project. Project Coordinator UU, herein supported by Catalyze, will ensure the efficient and effective coordination and management of the project.

