Reviewing Clinical Trial Trends in Tissue-Engineered Products
Chronic lower back pain is the leading cause of disability and morbidity worldwide. Degeneration of the spine’s intervertebral discs (IVDs) is a common cause of chronic lower back pain and accounts for about 40% of all cases. IVDs are structures that are embedded in the spine, and when healthy, they provide cushioning during movement. Current treatment options for lower back pain consists primarily of surgical interventions aimed at removing the damaged or altered tissue. However, surgery is not always a reliable option as success rates vary and the original ability to bear pressure (without injury) of the IVDs cannot be restored. Therefore, an alternative approach to treat IVD degeneration is necessary, with regenerative medicine and tissue engineering appearing particularly promising.
Advanced therapy medicinal products (ATMPs) are medicines for human use that are based on genes, tissues, and/or cells. They could offer opportunities for the treatment of diseases or injuries for which currently no sufficient treatment is available. Tissue-engineered products (TEPs) represent one of three distinct types of ATMPs. They can regenerate, repair and replace human tissue and are as such classified under regenerative medicine. These products are especially promising in degenerative musculoskeletal disorders such as intervertebral disc degeneration.
To bring new TEPs to patients, they need to be safe, efficacious and of high-quality standards. This is determined in a series of clinical trials which follow tight regulations. After demonstrating clinical success, the new TEPs can be granted market authorization by the European Medicines Agency and be utilised by patients. However, currently there is only little research performed into tissue engineered products. Literature shows that tissue-engineered products represented only 5% of all ATMPs in European clinical trials in 2019 and received only 5.1% of all financing for ATMPs. Instead, gene therapy products – another ATMP – were shown to take the lion’s share of funding and research.
As such, current trends in ATMP market approval have favoured gene therapy products, with a much smaller proportion of tissue engineered products being approved. Of the 21 total ATMPs that have been granted market authorization, only 4 have been TEPs.
Analysing trends in clinical trials investigating Tissue-Engineered Products
In January 2008, the European Parliament and Council of the European Union introduced a new regulation that defines how novel ATMPs can demonstrate quality, safety, efficacy, and good practice requirements necessary for market authorization. The regulation has proven effective at enhancing product development and successful use in clinical trials, but as previously mentioned, tissue engineered products represent a very small proportion of approved ATMPs. To uncover the reasons why, Kieran Joyce and colleagues determined how clinical trials using tissue engineered products between 2008 and 2021 were adapting to these new requirements. Some factors they looked at were how trials were financed, what pharmaceuticals were used, what route of administration they took, and what medical conditions were being treated, among others.
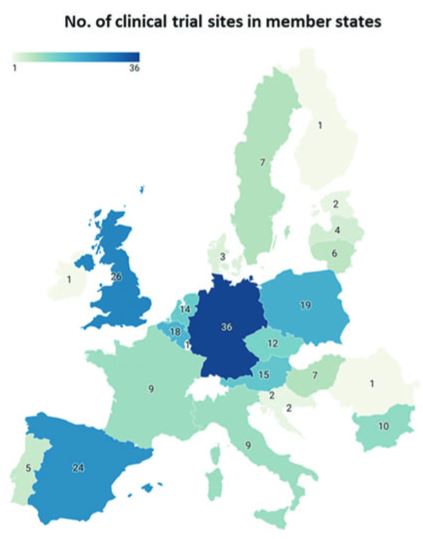
Geographical overview of clinical trial activity investigating TEPs in each Member State according to the EU Clinical Trials register
For clinical trials to run successfully, they needs to be financed. Financing a study is necessary and comes either from commercial or non-commercial partners, allowing it to eventually reach the market. Kieran and colleagues found that of the 90 trials identified, 63 were funded by commercial sponsors, representing a sizeable proportion of trial activity. This may be unsurprising as 30% of all trials conducted in this timeframe were late-stage trials that require ample financial investment, and present limited risk. However, commercial sponsors also accounted for one third more early-phase trials than their non-commercial counterparts. Usually, non-commercial support is more prevalent during early phase clinical trials where there is higher risk, but lower financial requirement. With a larger financial commitment from non-commercial partners such as national agencies and the EU, TEP investigation and subsequent market access could be improved.
Despite the intricacies surrounding TEP funding, clear progress has been made over the last 13 years. With the goal of maximising the potential for TEP market authorisation, there has been a clear shift in the cell source utilized in clinical trials. The shift from growing individual patient cells to batch processing donor cells has enabled a higher production rate for TEPs while reducing the risk of product rejection. This trend creates a low product variability with predictable potency – characteristics that makes early-stage clinical trials more attractive for non-commercial parties. This, coupled with a recent increase in funding of early-phase trials by non-commercial sponsors over the last two years, might indicate that TEPs may yet take a larger slice of ATMP market authorization.
With more early-phase trials listed in the coming years, it remains to be seen how upcoming trials align with the new regulations, to evaluate future TEPs with prospects of reaching the market.
Read about other therapies he
Issues with Tissues: Trends in Tissue-Engineered Products in Clinical Trials in the European Union
Kieran Joyce, MB BCh, BAO, PhD,* Zaklina Buljovcic, PhD,* Goran Rosic, PhD, Marietta Kaszkin-Bettag, PhD, and Abhay Pandit, PhD
First published: 21st October 2022
Abstract:
Tissue-engineered products (TEPs) consist of engineered cells or tissues produced to regenerate, repair, or replace
a dysfunctional, diseased, or absent human tissue. TEPs make up <5% of all advanced therapeutic medicinal
products (ATMPs) in clinical trials and received 5.1% of ATMP-designated funding in trials in the European
Union (EU) in 2019, highlighting the relatively low proportion of TEPs being developed. The realization of TEPs
being marketed has yet to be fulfilled, with few products being approved. Since 2009, 90 TEP-based clinical trials
have been undertaken in the EU. Of these 90, 25 were Phase I/II trials, 35 were Phase II, 28 were Phase III, and
two were Phase IV trials. This review provides an overview of TEPs in development, identifying musculoskeletal,
cardiovascular, and skin/connective tissue disorders as the main therapeutic areas of interest. Commercial
sponsors have funded most trials, and a significantly higher proportion of late-phase trials. Furthermore, this study has identified a shift toward the use of allogeneic cells in TEPs and increased activity in the proportion of early phase trials listed. This indicates a renewed interest in TEP development as sponsors adapt to the new regulation, with prospects of more TEP market authorization applications in the future.
Funding information: This publication has emanated from research conducted with the financial support of the College of Medicine, Nursing and Health Sciences, NUI Galway, Science Foundation Ireland (SFI), and the European Regional Development Fund (grant no. 13/RC/2073_P2) and the European Commission’s Horizon 2020 funding program for the iPSpine project (grant no. 825925).
Read the full paper here

