Understanding how material of healthy intervertebral disc cells can be used as therapy for back pain
Many individuals endure persistent lower back pain attributed to e.g. the natural wear and tear of intervertebral discs. Before these discs undergo deterioration, there’s a shift in the main cell types located in the nucleus pulposus, which is the central core of the disc. This transition involves a change from notochordal cells to nucleus pulposus cells.
The matrix (an extracellular substance where cells are embedded) produced by notochordal cells, present in youthful and healthy intervertebral discs, has demonstrated positive effects on degenerated human and canine intervertebral disc cells.
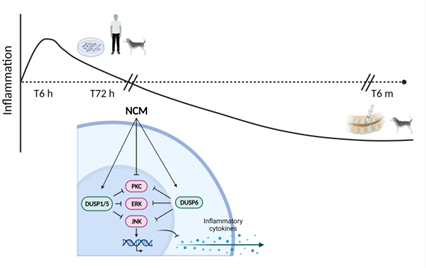
This figures shows the main conclusion of the study: NCM influences various signalling pathways within intervertebral disc cells. Although there was an initial short-term increase in inflammation, this was ultimately associated with a long-term decrease in inflammation.
Despite these benefits, the precise mechanism by which this notochordal cell-derived matrix exerts its effects remains unclear.
Addressing this knowledge gap, Lisanne Laagland and her colleagues sought to unravel how notochordal cell-derived matrix operates in the context of a degenerative intervertebral disc environment. Their research revealed that this matrix inhibits and influences various signalling pathways within intervertebral disc cells. Although there was an initial short-term increase in inflammation, this was ultimately associated with a long-term decrease in inflammation. Additionally, the matrix stimulated the expression of markers associated with healthy intervertebral disc cells.
These findings offer valuable insights into the mechanisms underlying the instructive capacity of notochordal cell-derived matrix. This knowledge has the potential to pave the way for a cell-free notochordal cell-based therapy, presenting a promising approach to address intervertebral disc degeneration.
Notochordal cell-derived matrix inhibits MAPK signaling in the degenerative disc environment
L.T. Laagland, F.C. Bach, F.M. Riemers, G. Erdmann, T.S. Braun, G.G.H. van den Akker, T.C. Schmitz, L.B. Creemers, C. Sachse, C.L. Le Maitre, T.J.M. Welting, K. Ito, M.F. Templin and M.A. Tryfonidou
First published : 7 November 2023
Abstract:
Chronic low back pain is often caused by intervertebral disc (IVD) degeneration. Preceding this degenerative process, the main cellular phenotype in the nucleus pulposus shifts from notochordal cells (NCs) to nucleus pulposus cells (NPCs). In previous studies, porcine NC-derived matrix (NCM), containing NC-secreted factors, induced matrix anabolic effects and inhibited pro-inflammatory mediators in NPCs in vitro and in degenerated canine IVDs in vivo. As the underlying mechanisms remained elusive, this study aimed to explore this with targeted gene expression and proteomic (DigiWest technology) analysis focused on inflammatory signaling pathways.
After 6 hours, NCM (10 mg/mL) treatment initially stimulated pro-inflammatory mediators in canine and human NPCs in vitro and increased signaling in a chondrosarcoma derived Nuclear factor-κB reporter cell line. At protein level, NCM mainly induced changes in the Mitogen-activated protein kinase (MAPK) pathway after 72 hours. Expression of inhibitory Dual-specificity phosphatase (DUSP) proteins was increased in NCM-treated NPCs, whereas the expression of the three main pillars of the MAPK pathway (extracellular signal-regulated kinase (ERK)/cJun N-terminal kinase (JNK)/protein kinase C (PKC)) was inhibited. In follow-up validation, in vivo degenerated canine IVDs treated with an intradiscal NCM injection demonstrated increased DUSP5 and healthy nucleus pulposus marker (cytokeratin 19, Paired box 1 (PAX1), Forkhead Box F1 (FOXF1)) immunopositivity after 6 months of treatment.
Altogether, NCM initially stimulated pro-inflammatory mediators in vitro, but thereafter exerts its prolonged effects by inhibiting the MAPK pathway. These findings provide insights in the underlying mechanisms involved in the instructive capacity of this naturally-derived biomaterial with the potential to serve as a cell-free NC-based therapy to treat intervertebral disc degeneration.
Funding information: Dutch Arthritis Society, Grant/AwardNumbers: LLP12, LLP22; Horizon 2020 Framework Programme, Grant/AwardNumber: 825925
Read the full publication here: article

